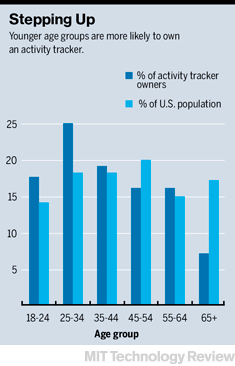
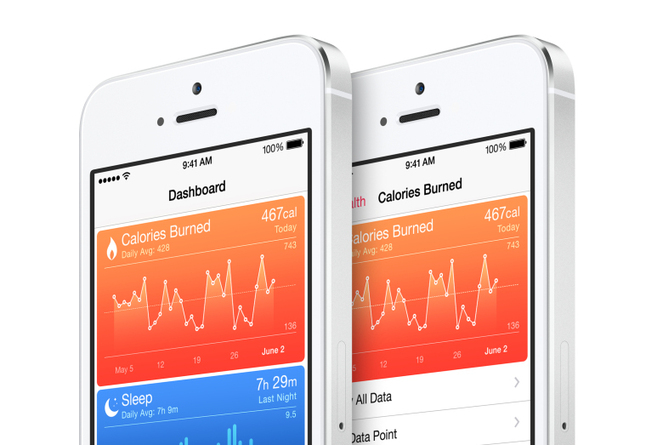
Among technologists, mobile health is thriving. Since the start of 2013, more than $750 million in venture capital has been invested in companies that do everything from turn your smartphone into a blood pressure gauge to snapping medical–quality images of the inner ear. Apple, Qualcomm, Microsoft, and other corporate giants are creating mobile health products and investing in startups.
Source: www.technologyreview.com
It's been tough to identify the problems that only turn up after medicines are on the market. An experimental project is now combing through data to get earlier, more accurate warnings.
No one likes it when a new drug in people's medicine cabinets turns out to have problems — just remember the Vioxx debacle a decade ago, when the painkiller was removed from the market over concerns that it increased the risk of heart attack and stroke.
To do a better job of spotting unforeseen risks and side effects, the Food and Drug Administration is trying something new — and there's a decent chance that it involves your medical records.
It's called Mini-Sentinel, and it's a $116 million government project to actively go out and look for adverse events linked to marketed drugs. This pilot program is able to mine huge databases of medical records for signs that drugs may be linked to problems.
The usual system for monitoring the safety of marketed drugs has real shortcomings. It largely relies on voluntary reports from doctors, pharmacists, and just plain folks who took a drug and got a bad outcome.
"We get about a million reports a year that way," says Janet Woodcock, the director of the FDA's Center for Drug Evaluation and Research. "But those are random. They are whatever people choose to send us."
Source: www.npr.org
The Food and Drug Administration (FDA), which regulates everything from heart monitors to horse vaccines, will soon have its hands full with consumer health apps and devices.
The vast majority of the health apps you’ll find in Apple’s or Google’s app stores are harmless, like step counters and heart beat monitors. They’re non-clinical, non-actionable, and informational or motivational in nature.
But the next wave of biometric devices and apps might go further, measuring things like real-time blood pressure, blood glucose, and oxygen levels.
More clinical apps
The FDA is charged with keeping watch on the safety and efficacy of consumer health products. Lately, that includes more clinical apps as well as devices you might buy at the drugstore, like a home glucose testing kit.
“It’s these apps that the FDA says it will regulate,” David Bates of Brigham and Women’s Hospital and Physicians Organization told VentureBeat in June. These apps will have to go through the full 510(k) process,” he said.
Dr. Bates chaired a group to advise the FDA on how to review health apps for approval, and on how the FDA should advise developers.
“It was intended to help them think through the risk factors involved with these products and then give guidance on how to stay within the guidelines,” he said.
“The device makers were asking from some guidance from The FDA on what types of things would be accepted and what wouldn’t,” Bates said.
Bates believes the FDA wants to use a light regulatory touch when looking at new medical devices. “The FDA definitely wants innovation to continue in clinical devices,” he said. “In general the FDA knows that the vast majority of apps are just informational.”
The FDA’s final guidance focuses on a small subset of mobile apps that present a greater risk to patients if they do not work as intended.
Health apps go mainstream
The big software companies (Apple, Google, and Samsung) have brought attention to, and lent credibility to, apps and devices that do more than count steps. These companies are building large cloud platforms designed to collect health data from all sorts of health apps and devices.
more at http://venturebeat.com/2014/07/21/health-apps-are-changing-so-must-the-fda/
Source: venturebeat.com
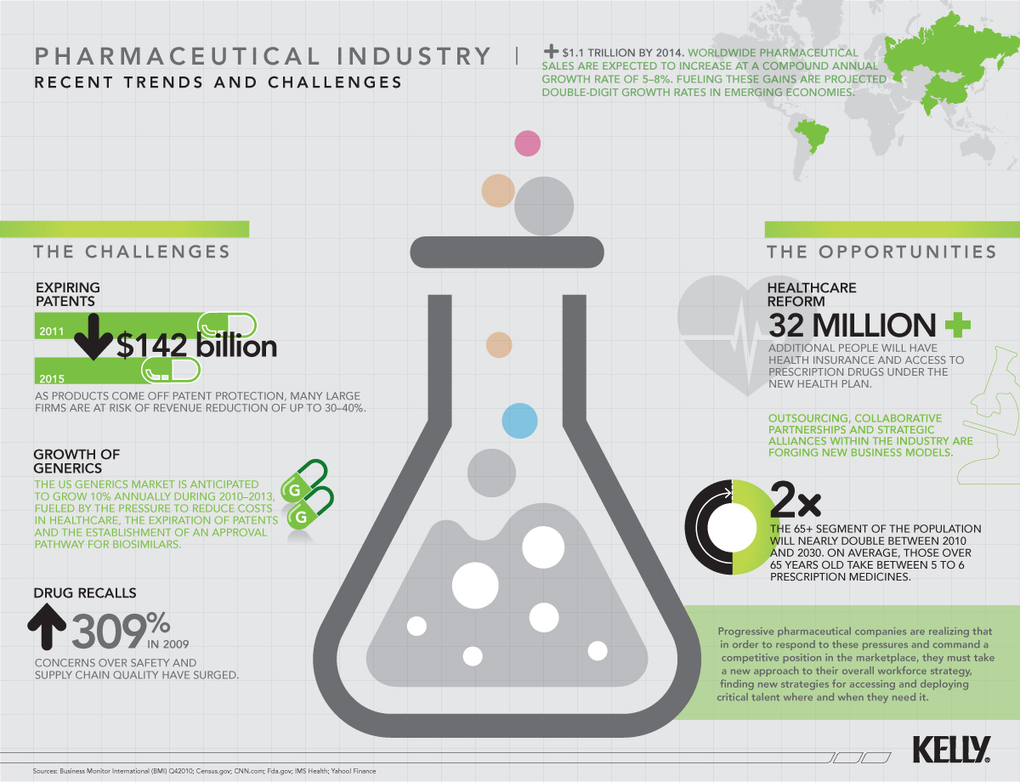
Digital Transformation in the pharmaceutical industry provides the means to, not only revise its business model, but also increase its relevance to its ultimate customers – patients, physicians and other consumers of its products and services.
Source: smartdatacollective.com